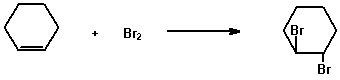
There is no definite set procedure that can be generally applied to organic qualitative analysis. Various books have different approaches, but a systematic approach based on the scheme given below will give good results.
Students should, however, consult the laboratory manual and Textbook of Practical Organic Chemistry, A.I. Vogel (4th Edition).
(a) Quantities of substance for tests. For most tests about 0.1 g solid or 0.1 - 0.2 mL (2 - 3 drops) of liquid material (NOT MORE) should be used.
(b) Reagents likely to be met within organic analysis are on the reagent shelves. Students are advised to develop a general knowledge of the physical characteristics of common organic compounds. If in doubt about the expected result of a test between a certain compound and a reagent, carry out a trial test with a known compound and compare with the unknown.
(c) Quantities of substance derivatives. Students have wasted much time and material in the past by taking too large a quantity of substance for preparation of a derivative. In general, 0.5 - 1 g (or 0.5 - 1 mL) of substance gives the most satisfactory results.
If a practical book instructs one to use larger quantities (3 - 4 g or more), the quantities should be scaled down to 1 g or 1 mL of the unknown substance and corresponding quantities of reagents should be used.
(a) Note physical characteristics - solid, liquid, colour and odour.
(b) Perform an ignition test (heat small amount on metal spatula) to determine whether the compound is aliphatic or aromatic (i.e. luminous flame - aliphatic; sooty flame - aromatic).
B. Physical Constants
Determine the boiling point or melting point. Distillation is recommended in the case of liquids (see Appendix 3). It serves the dual purpose of determining the b.p., as well as purification of the liquid for subsequent tests.
C. Analysis for elements present
At C10 level, the elements present will be told to you, but read up the method.
D. Solubility tests
The solubility of the unknown in the following reagents provides very useful information. In general, about 3 mL of the solvent is used with 0.1 g or 0.2 mL (2 - 3 drops) of the substance. The class of compound may be indicated from the following table:
| REAGENT AND TEST | CLASS | GROUP OF COMPOUNDS |
| Soluble in cold or hot water. (If the unknown is soluble do NOT perform solubility tests below) | Neutral, acidic or basic. (Test with litmus or universal indicator paper) | Lower members of series. Neutral, e.g. alcohols; Acidic, e.g. acids, phenols; Basic, e.g. amines | Soluble in dil. HCl | Basic | Most amines (except III amines with only aromatic groups |
| Soluble in dil. NaOH | Acidic | Most acids, most phenols. |
| Soluble in NaHCO3 | Strongly acidic | Most carboxylic acids. |
| Insoluble in water, acid and alkali | Neutral | Hydrocarbons, nitrohydro-carbons, alkyl or aryl halides, esters and ethers. Higher molecular weight alcohols, aldehydes and ketones |
From the previous tests it is often possible to deduce the functional groups present in the unknown compound. Consult i.r. spectra when available.
Individual tests are then performed to identify and confirm the functional groups present.
2. A systematic approach cannot be overemphasised in group classification tests to avoid confusion and error.
F. Consultation of Literature
Once the functional group has been identified, reference is made to tables in a book on organic analysis, for assessing possibilities and for the preparation of suitable solid derivatives.
It should be noted that whilst two substances with the same functional group may sometimes have very similar b.p. or m.p., solid derivatives canusually be chosen from the literature, with m.p. differences of about 10 (or more), which distinguish between the two possibilities.
Example:
COMPOUND B.P. DERIVATIVES (M.P.)
2,4-DNPH SEMICARBAZONE
Diethyl ketone 102 156 139
Methyl n-propyl ketone 102 144 112
G. Preparation of derivatives
The final characterisation of the unknown is made by the preparation of suitable solid derivatives. The derivative should be carefully selected and its m.p. should preferably be between 90 - 150 for ease of crystallisation and m.p. determination.
Preparation of one derivative should be attempted. The derivative should be purified by recrystallisation, dried and the m.p. determined. Derivatives should be submitted correctly labelled for assessment together with the record.
Recording of Results
The results should be recorded in a systematic manner. Results should be recorded in the practical book at the time (not written up afterwards).
A record should be made of every test carried out, no matter whether a NEGATIVE RESULT HAS BEEN OBTAINED.
Test, observation and inference should be given.
At the conclusion of the analysis a brief summary of results should be included, giving the name, b.p. or m.p., and formula of the analysed compound.
Qualitative Analysis for Elements (for reference only)
In organic compounds the elements commonly occurring along with carbon and hydrogen, are oxygen, nitrogen, sulphur, chlorine, bromine and iodine. The detection of these elements depends upon converting them to water-soluble ionic compounds and the application of specific tests.
Lassaigne's Sodium Fusion Test
C, H, O, N, S, X NaX
NaCN
-> Na2S
NaCNS
PROCEDURE
Place a piece of clean sodium metal, about the size of a pea into a fusion tube. Add a little of the compound (50 mg or 2 - 3 drops).* Heat the tube gently at first, allowing any distillate formed to drop back onto the molten sodium. When charring begins, heat the bottom of the tube to dull redness for about three minutes and finally plunge the tube, while still hot, into a clean dish containing cold distilled water (6 mL) and cover immediately with a clean wire gauze.**
*For liquids it is better to first melt the sodium add the liquid drop by drop.
**CAUTION: The tube shatters, and any residual sodium metal reacts with water. Stir the mixture, boil for 1 - 2 minutes, on a tripod and filter hot through a fluted paper.
The 'fusion' filtrate which should be clear and colourless, is used for the SPECIFIC TESTS DESCRIBED BELOW:
1. To a portion (2 mL) of the 'fusion' filtrate add 0.2 g of powdered ferrous sulphate crystals. Boil the mixture for a half a minute, cool and acidify by adding dilute sulphuric acid dropwise. Formation of a bluish-green precipitate (Prussian blue) or a blue solution indicates that the original substance contains nitrogen. If no precipitate appears, allow to stand for 15 minutes, filter and inspect filter paper.
2. SULPHUR (SULPHIDE)
To the cold 'fusion' filtrate (1 mL) add a few drops of cold,
freshly prepared, dilute solution of sodium nitroprusside. The
latter may be prepared by adding a small crystal of the solid to
2 mL of water. Production of a rich purple colour indicates that
the original substance contains sulphur. This test is very
sensitive. Only strong positive results are significant.
3. HALOGENS (HALIDES)
Acidify a portion (1 mL) of the 'fusion' filtrate with 2N nitric
acid, and if nitrogen and/or sulphur are present, boil for 1 - 2
minutes.* Cool and add aqueous silver nitrate (1 mL), compare
with a blank. Formation of a heavy, white or yellow precipitate
of silver halide indicates halogen. If a positive result is
obtained: acidify the remaining portion of the 'fusion' filtrate
with dilute sulphuric acid, boil and cool. Add carbon
tetrachloride (1 mL) and a few drops of freshly prepared chlorine
water. Shake the mixture.
(a) If the carbon tetrachloride layer remains colourless - indicates chlorine.
(b) If the carbon tetrachloride layer is brown - indicates bromine.
(c) If the carbon tetrachloride layer is violet - indicates iodine.
*If nitrogen and/or sulphur are also present, the addition of silver nitrate to the acidified 'fusion' solution will precipitate silver cyanide and/or silver sulphide in addition to the silver halides. The removal of hydrogen cyanide and/or hydrogen sulphide is effected by boiling the 'fusion' solution. GROUP CLASSIFICATION TESTS
Some functional group tests are listed below. Students should refer to a practical text book for details, and further information, e.g. Vogel.
Tests for unsaturation
1. Cold dilute potassium permanganate solution.
2. Solution of bromine in carbon tetrachloride.
Tests for compounds containing nitrogen
1. Amines
(a) Nitrous acid.
(b) Confirmatory tests.
2. Compounds which give amines or ammonia on acid or alkaline
hydrolysis:
Amides, substituted amides, anilides, nitriles.
3. Compounds which give amines on reduction:
Nitro, nitroso, azo, hydrazo, nitriles.
Tests for compounds containing C, H and possibly oxygen
1. Carboxylic acids
Na2CO3 or NaHCO3 solution liberate carbon dioxide.
2. Phenols
(a) Sodium hydroxide solution (soluble). Insoluble in and no CO2
from NaHCO3 (except when electron attracting groups present, e.g.
2,4-dinitrophenol).
(b) Ferric chloride solution.
(c) Bromine water.
3. Aldehydes and Ketones
(a) 2,4-dinitrophenylhydrazine (as Brady's reagent) for
C=O.
(b) Iodoform test for CH3CO-.
4. Aldehydes only (reducing properties)
(a) Fehling's solution.
(b) Tollen's reagent (ammoniacal AgNO3 solution).
(c) Jones reagent.
5. Alcohols
(a) Lucas' reagent to distinguish I, II and III alcohols.
(b) Jones reagent.
(c) Metallic sodium (use dry liquid and dry tube).
6. Sugars
(a) Molisch's test.
7. Esters
(a) Hydroxamic acid test.
(b) Hydrolysis.
Date......................................
Compound containing C, H (N, Hal, S)
Physical characteristics ...................... (solid, liquid, gas, colour, odour, etc.)
Ignition test .............................. (aromatic or aliphatic)
Physical constant ........................ (boiling point or melting point)
Solubility tests (in tabular form)
Group classification tests (in tabular form)
Test Observation Inference
From the above tests and observations the given compound is probably a
.........................(acid, phenol, aldehyde, etc.)
Consultation of literature (Possibilities) M.P. of derivative
(a)
(b)
(c)
Preparation of derivative (method of preparation)
Observed m.p. of derivative
Lit. m.p. of derivative
Result
Compound No. ........................ is ............................
(give formula)
Two common types of unsaturated compounds are alkenes and alkynes characterised by the carbon-carbon double and triple bond, respectively, as the functional group. The two common qualitative tests for unsaturation are the reactions of the compounds with (a) bromine in carbon tetrachloride and (b) potassium permanganate.
(a) 2% Bromine in carbon tetrachloride
Dissolve 0.2 g (or 0.2 mL) of the compound in 2 mL of carbon
tetrachloride or another suitable solvent and add the solution
dropwise to 2 ml of 2% bromine solution in carbon tetrachloride
and shake.
e.g.
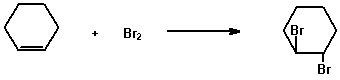
Rapid disappearance of the bromine colour to give a colourless solution is a positive test for unsaturation.
NOTE: The reagent is potentially dangerous. Keep it
off your skin and clothes; protect your eyes and nose.
(b) 2% Aqueous potassium permanganate
Dissolve 0.2 g (or 0.2 mL) of the substance in 2 mL of water
(acetone may also be used as solvent). Add the potassium
permanganate solution dropwise and observe the result.
e.g.
For a blank determination, count the number of drops added to 2 mL of acetone before the colour persists. A significant difference in the number of drops required in the two cases is a positive test for unsaturation.
II. COMPOUNDS CONTAINING NITROGEN
1. Amines
(a) Reaction with nitrous acid Dissolve the amine (0.5 mL) in
concentrated acid (2.0 mL) and water (3 mL) and cool the solution
to 0 - 5 in an ice-bath for 5 minutes. Add a cold solution
(ice-bath) of sodium nitrite (0.5 g) in water (2.0 mL) from a
dropper, with swirling of the test tube, still keeping the
mixture in the ice-bath.
AMINE REACTION
I aliphatic N2 evolved.
RNH2 + HNO2 -> ROH + N2 + H2O
__________________________________________________________________
I aromatic Diazonium salt is formed.
ArNH2 + HNO2 -> ArN=N+
Add the cold diazonium solution and with swirling
to a cold solution of 2-naphthol (0.2 g) in 5% NaOH
solution (2 mL). An orange-red azo dye is formed.
__________________________________________________________________
II aliphatic and Yellow oily nitrosamines are generally formed.
II aromatic R2NH + HNO2 -> R2N-NO
__________________________________________________________________
III aliphatic No visible reaction.
__________________________________________________________________
III aromatic Dialkylanilines yield green solid p-nitroso
compounds (if p-position unsubstituted).
__________________________________________________________________
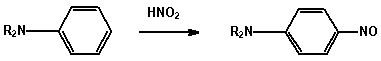
e.g. (a) C6H5SO2Cl + H-NHR + NaOH -> C6H5SO2NHR + NaCl + H2O (b) C6H5SO2Cl + H-NR2 + NaOH -> C6H5SO2NR2 + NaCl + H2OThe substituted sulphonamide formed from a primary amine dissolves in the alkali medium whilst that produced from a secondary amine is insoluble in alkali.
Place 0.5 mL (or 0.5 g) of the compound, 15 - 10 mL of 5% NaOH and 1 mL of benzenesulphonyl chloride in a test tube, stopper the tube and shake until the odour of the sulphonyl chloride has disappeared. The solution must be kept alkaline (if no reaction has occurred, the substance is probably a tertiary amine).
If a precipitate appears in the alkaline solution, dilute with about 10 mL of water and shake; if the precipitate does not dissolve, a secondary amine is indicated.
If there is no precipitate, acidify it cautiously to congo red with concentrated hydrochloric acid (added dropwise): a precipitate is indicative of a primary amine.
2. Amides R-CO-NH2
Simple primary amides can be decomposed by boiling with alkali
and thereby evolving ammonia.
e.g. CH3-CO-NH2 + NaOH -> CH3-CO2- Na+ + NH3 �
Boil 0.5 g of the compound with 5 mL of 10% sodium hydroxide
solution and observe whether ammonia is evolved.
III. COMPOUNDS CONTAINING C, H AND POSSIBLY OXYGEN
1. Carboxylic acids - test with 5% aq. NaHCO3
R-CO2H + NaHCO3 -> R-CO2- Na+ + CO2 � + H2OSodium hydrogen carbonate reacts with carboxylic acids to give the sodium salt of the acid and liberates carbon dioxide. If the acid is insoluble in water and the reaction is sluggish dissolve the acid in methanol and add carefully to a saturated sodium hydrogen carbonate solution, when a vigorous effervescence will be observed.
2. Phenols [Soluble in NaOH and produce no CO2 from
NaHCO3]
(a) Bromine water
Phenols are generally highly reactive towards electrophilic
reagents and are readily brominated by bromine water. e.g.
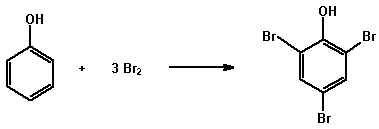
Dissolve or suspend about 0.05 g of the compound in 2 mL of dilute hydrochloric acid and add bromine water dropwise until the bromine colour remains. A white precipitate of the bromophenol may form. Solid bromophenol derivatives can be used for the confirmation of the structure of a phenol (cf the preparation of derivatives).
(b) Ferric chloride test
Most phenols react with iron (III) chloride to form coloured
complexes. The colours vary - red, purple, blue or green -
depending on various factors, e.g. the phenolic compound used,
the solvent, concentration. Since some phenols do not give
colours, a negative test must not be taken as significant without
supporting information.
Dissolve 0.05 g of the compound in 2 mL water (or a mixture of water and ethanol if the compound is not water-soluble) and add an aqueous solution of ferric chloride dropwise. Observe any colour changes which may occur.
3. Aldehydes and ketones
(a) 2,4-Dinitrophenylhydrazine (as Brady's reagent) A test for
the carbonyl group (C=O) in aldehydes and ketones.
2,4-Dinitrophenylhydrazine gives sparingly soluble yellow or red
2,4-dinitrophenylhydrazones with aldehydes and ketones.
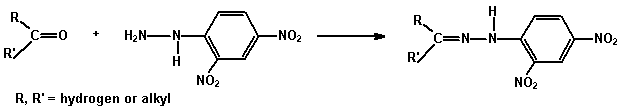
Add 3 mL of the reagent to 2 drops of the compound in a test tube and shake. If no precipitate forms immediately, warm and allow to stand for 5 - 10 minutes. A crystalline precipitate indicates the presence of a carbonyl compound.
The bench reagent is very dilute and is intended for
qualitative tests only and should not be used in the preparation
of a derivative for identification purposes. (b) Iodoform test
for CH3CO-
Dissolve 0.1 g (or 5 drops) of the compound in 2 mL of water; if
it is insoluble in water add sufficient dioxan to produce a
homogeneous solution. Add 2 mL of 5% NaOH solution and then
introduce the potassium iodide - iodine reagent dropwise with
shaking until a definite dark colour of iodine persists. Allow to
stand for 2 - 3 minutes; if no iodoform separates at room
temperature, warm the test tube in a beaker of water at 60 . Add
a few more drops of the iodine reagent if the faint iodine colour
disappears. Continue the addition of the reagent until a dark
colour is not discharged after 2 minutes heating at 60 . Remove
the excess of iodine by the addition of a few drops of dilute
sodium hydroxide solution with shaking, dilute with an equal
volume of water, and allow to stand for 10 minutes. The test is
positive if a yellow precipitate of iodoform is deposited. Filter
off the yellow precipitate, dry upon pads of filter paper and
determine the m.p. Iodoform melts at 120 (it can be
recrystallised from methanol- water).
The reaction is given by acetaldehyde and simple methyl ketones. Alcohols containing the CH3CHROH group will be oxidised under the reaction conditions and also give a positive test.
4. Aldehydes only (reducing properties).
(a) Fehling's solution
Aldehydes reduce Fehling's solution to yellow or red copper (I)
oxide.
Preparation of the reagent: Mix equal volumes of Fehling's solution solution I (aqueous alkaline potassium tartrate) and Fehling's solution II (copper sulphate solution).
Add 2 drops (or 0.05 g) of the compound and 2 - 3 drops of the reagent and heat on a boiling water bath for 3 - 4 minutes.
The test is positive for aliphatic aldehydes, but is often indecisive for aromatic aldehydes where Jones' Reagent is often useful (see 5).
(b) Tollen's reagent (Ammonical silver nitrate solution)
Aldehydes are readily oxidised to carboxylic acids and will
reduce Tollen's reagent to produce a silver mirror on the inside
of a clean test tube.
FIRST clean up a test tube with a little hot nitric acid (fume cupboard) and rinse with distilled water.
Preparation of the reagent: To 1 mL of silver nitrate solution add a few drops of sodium hydroxide. Then add dilute ammonium hydroxide dropwise until the precipitate just dissolves.
Add 2 - 3 drops of the compound in methanol to 2 - 3 mL of Tollen's solution contained in a very clean test tube. If no reaction takes place in the cold, warm gently in a water bath.
CAUTION: After the test, pour the contents of the test tube into the sink and wash the test tube with dilute nitric acid. Any silver fulminate present, which is highly explosive when dry, will be destroyed.
(c) Jones Reagent (See section under alcohols).
5. Alcohols
The tests for the hydroxyl group not only detect the presence of
the group, but may also indicate whether it is primary, secondary
or tertiary.
(a) Jones Reagent (CrO3-H2SO4
in H2O)
This reagent distinguishes primary and secondary alcohols from
tertiary alcohols; the test is based on the much greater
resistance to oxidation of tertiary alcohols compared to the
other two types. Aldehydes also give a positive test.
Place 1 mL of acetone in a test tube and dissolve one drop of
a liquid or ca 10 mg of a solid alcohol or aldehyde in it. Add
one drop of the reagent to the acetone solution and shake the
tube to mix the contents. Primary and secondary alcohols react
within two seconds as indicated by the disappearance of the
orange colour of the reagent and the formation of a green or
blue-green precipitate or emulsion.
Tertiary alcohols do not react even after 3 minutes.
(I) RCH2OH -> RCHO -> RCO2H (II) R2CHOH -> R2C=O (III) R3COH -> no visible reaction.
(b) Lucas' Reagent [ZnCl2 - conc. HCl]
This reagent converts alcohols into the corresponding alkyl
chlorides. Zinc chloride (a Lewis acid) increases the reactivity
of alcohols towards acid. The test depends on the rate of
reaction of primary, secondary, and tertiary alcohols with the
reagent at room temperature.
(I) RCH2OH -> no reaction at room temperature. (II) R2CHOH -> R2CHCl + H2O (1 hour or maybe longer) (III) R3COH -> R3CCl + H2O (immediately)To 1 mL of the alcohol in a small test tube add 6 mL of Lucas' reagent at room temperature. Close the tube with a cork, shake and allow to stand.
(i) Primary alcohols - the aqueous phase remains clear (except allyl alcohol - droplets after 7 minutes).
(ii) Secondary alcohols - very slow reaction (~ 1 hour or maybe longer) when droplets of alkyl chloride may be seen.
(iii) Tertiary alcohols - very fast reaction and droplets of the alkyl chloride formed almost immediately.
6. Sugars, Carbohydrates
Molisch's Test
This is a general test for carbohydrates. Dissolve 20 - 30 mg of
the compound in 2 mL water and add 0.5 mL of the reagent (a 20%
solution of 2-naphthol in ethanol). Pour 2 mL of concentrated
sulphuric acid from a dropper carefully down the side of the tube
so that the acid forms a layer beneath the aqueous solution
without mixing with it. A red colouration, changing to dark
purple forms at the interface. Carry out a second test on a blank
solution.
7. Esters
Hydroxamic acid test
R-CO-OR' + H2N-OH -> R-CO-NH-OH + R'-OH
Esters react with hydroxylamine in the presence of sodium hydroxide to form the sodium salt of the corresponding hydroxamic acid. On acidification and addition of ferric chloride the magenta-coloured iron (III) complex of the hydroxamic acid is formed.
It is always advisable to ensure that an unknown compound does not give a colour with iron (III) chloride before carrying out the hydroxamic acid test.
Procedure for hydroxamic acid test
(a) Ferric chloride test
Dissolve a drop or a few small crystals of the compound in 1 mL
of 95% ethanol (rectified spirit) and add 1 mL of M hydrochloric
acid. Note the colour produced when 1 drop of 5% iron (III)
chloride is added to the solution. If a pronounced violet, blue,
red or orange colour is produced, the hydroxamic acid test
described below is NOT APPLICABLE.
(b) Hydroxamic acid test
Mix 1 drop or several small crystals (ca 0.05 g) of the compound
with 1 mL of 0.5 M hydroxylamine hydrochloride in 95% ethanol and
add 0.2 mL of 6 M aqueous sodium hydroxide. Heat the mixture to
boiling and after the solution has cooled slightly add 2 mL of M
hydrochloric acid. If the solution is cloudy, add 2 mL of 95%
ethanol. Observe the colour produced when 1 drop of 5% iron (III)
chloride solution is added. If the resulting colour does not
persist, continue to add the reagent dropwise until the observed
colour pervades the entire solution. Usually only 1 drop of the
iron (III) chloride solution is necessary. Compare the colour
with that produced in test (a). A positive test will be a
distinct burgundy or magenta colour as compared with the yellow
colour observed when the original compound is tested with iron
(III) chloride solution in the presence of acid. It is often
advisable to conduct in parallel the test with, say, ethyl
acetate, to ensure that the conditions for this test are
correct.
The following table lists some of the classes of organic compounds and a selection of derivatives that may be prepared to characterise them. Check with the tables of melting points in Vogel which derivatives are most suitable for the characterisation of your particular compound.
| CLASS OF COMPOUND | DERIVATIVES |
| 1. ALCOHOLS | 3,5-dinitrobenzoate |
| 2. PHENOLS | benzoate, acetate, bromo-derivative |
| 3. ALDEHYDES AND KETONES | semicarbazone, 2,4-dinitrophenyl-hydrazone, oxime |
| 4. ACIDS | anilide, amide, p-toluidide. |
| 5. AMINES | benzoyl, acetyl and sulphonamide derivatives |
ALCOHOLS
(i) 3,5-Dinitrobenzoates
3,5-Dinitrobenzoyl chloride is usually partially hydrolysed and
should be prepared in the pure state by heating gently a mixture
of 3,5-dinitrobenzoic acid (1 g) and phosphorus pentachloride
(1.5 g) in a dry test tube, until it liquifies (5 min).* The
liquid is poured on a dry watch glass and allowed to solidify.
The phosphoryl chlorides are removed by pressing the solid with a
spatula on a wad of filter paper. The residual acid chloride is
suitable for immediate use in the preparation of the
derivatives.
*Work under fume hood. Fumes are irritating to the eyes and nose.
The 3,5-dinitrobenzoyl chloride is mixed with the alcohol (0.5 - 1 mL) in a loosely corked dry test tube and heated on a steam bath for about 10 min. Secondary and tertiary alcohols require up to 30 min. On cooling add 10 mL sodium hydrogen carbonate solution, stir until the ester crystallises out, and filter at the pump. Wash with a little carbonate solution, water and suck dry. Recrystallise from the minimum hot ethanol or light petroleum. Cool slowly to avoid the formation of oily droplets of your ester.
PHENOLS
(i) Benzoates (Sch�tten-Baumann method).
To the phenol (0.5 g) is added 5% sodium hydroxide (10 mL) in a
well-corked boiling tube or a small conical flask. Benzoyl
chloride (2 mL) is added in small quantities at a time, and the
mixture shaken vigorously with occasional cooling under the tap
or in ice-water. After 15 min the solid benzoate separates out:
the solution should be alkaline at the end of the reaction; if
not alkaline, or if the product is oily, add a solid pellet of
sodium hydroxide and shake again. Collect the benzoate, wash
thoroughly with cold water, and recrystallise from alcohol or
light petroleum.
(ii) Acetates
Acetates of many simple phenols are liquids; however, this is a
suitable derivative for polyhydric and substituted phenols. The
phenol (0.5 g) is dissolved in 10% sodium hydroxide solution and
an equal quantity of crushed ice is added, followed by acetic
anhydride (2 mL). The mixture is vigorously shaken in a stoppered
test tube until the acetate separates. The product is filtered
and recrystallised from alcohol.
(iii) Bromo derivatives
The phenol (0.3 g) is suspended in dilute hydrochloric (10 mL)
and bromine water added dropwise until no more decolourisation
occurs. The bromo derivative which precipitates out is filtered
off and recrystallised from alcohol.
ALDEHYDES AND KETONES
(i) Semicarbazones
Dissolve semicarbazide hydrochloride (1 g) and sodium acetate
(1.5 g) in water (8 - 10 mL), add the aldehyde or ketone (0.3 mL)
and shake. Shake the mixture for a few minutes and then cool in
ice-water. Filter off the crystals, wash with a little cold water
and recrystallise from methanol or ethanol.
(ii) 2,4-Dinitrophenylhydrazones
Suspend 0.25 g of 2,4-dinitrophenylhydrazine in 5 mL of methanol
and add 0.5 mL of concentrated sulphuric acid cautiously. Filter
the warm solution and add a solution of 0.2 g of the carbonyl
compound in 1 mL of methanol. Recrystallise the derivative from
methanol, ethanol or ethyl acetate.
(iii) Oximes
Hydroxylamine hydrochloride (0.5 g) is dissolved in water (2
mL). 10% sodium hydroxide (2 mL) and the carbonyl compound (0.2 -
0.3 g) dissolved in alcohol (1 - 2 mL) are added, the mixture
warmed on a steam bath for 10 min and then cooled in ice.
Crystallisation is induced by scratching the sides of the test
tube with a glass rod. The oximes may be crystallised from
alcohol.
ACIDS
(i) Amides, anilides and p-toluidides
The acid (0.5 g) is refluxed with thionyl chloride (2 - 3 mL) in
a fume cupboard for about 30 mins.* It is advisable to place a
plug of cotton wool in the top of the reflux condenser to exclude
moisture. The condenser is removed and the excess of thionyl
chloride is distilled off (b.p. 78 ). The acid chloride thus
produced is treated with concentrated ammonia solution (5 mL) or
aniline (0.5 - 1 mL) or p-toluidine (0.5 - 1 g), when the solid
derivative separates out. It is collected and recrystallised from
alcohol adding decolourising charcoal if found necessary.
*Alternately use PCl5 to form the acid chloride.
AMINES
(i) Acetyl derivatives (acetamides)
Reflux gently in a small dry flask under a dry condenser the
amine (1 g) with acetic anhydride (3 mL) for 15 min. Cool the
reaction mixture and pour into 20 mL cold water. Boil to
decompose the excess acetic anhydride. Cool and filter by suction
the insoluble derivative. Recrystallise from ethanol.
(ii) Benzoyl derivatives (benzamides)
Suspend 1 g of the amine in 20 mL of 5% aqueous sodium hydroxide
in a well-corked flask, and add 2 mL benzoyl chloride (fume
hood!), about 0.5 mL at a time, with constant shaking. Shake
vigorously for 5 - 10 min until the odour of the benzoyl chloride
has disappeared. Ensure that the mixture remains alkaline. Filter
off the solid derivative, wash with a little cold water and
recrystallise from ethanol.
(iii) Benzenesulphonamides
To 1 g of the amine in 20 mL of 5% sodium hydroxide solution in
a well-corked flask add 1 mL benzenesulphonyl chloride (fume
hood!). Shake the mixture until the odour of the sulphonyl
chloride disappears. Check that the solution is alkaline. Acidify
if necessary to obtain the precipitated derivative. Concentrated
hydrochloric acid added dropwise should be used. Filter the
product, wash with a little cold water and suck dry.
Recrystallise from ethanol.
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page Copyright © 2005 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,