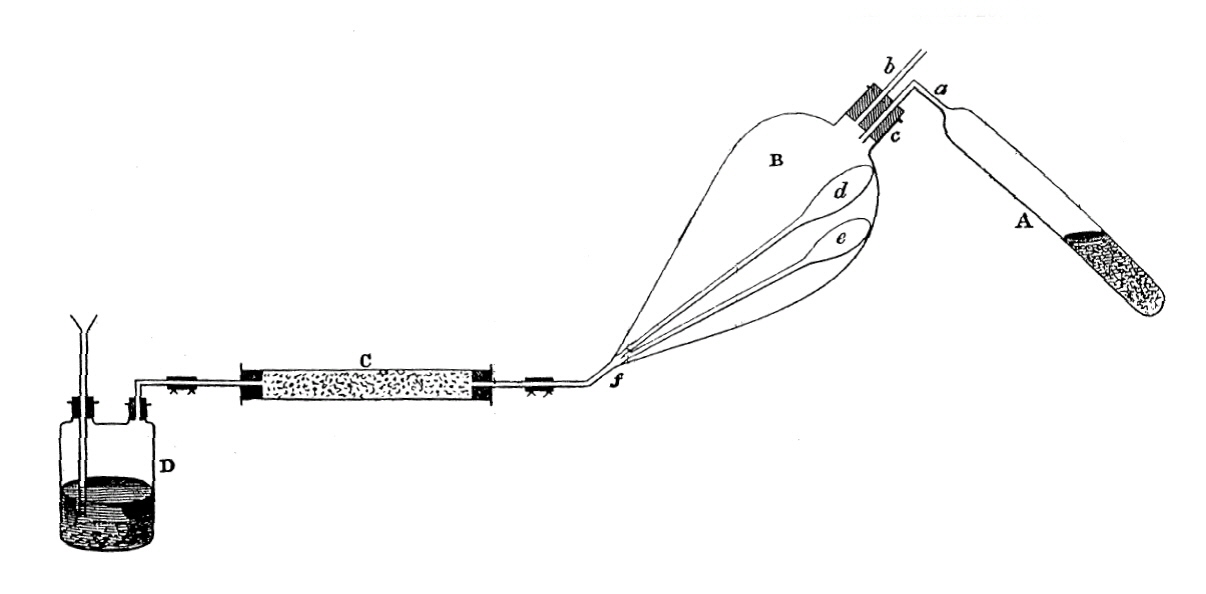
Apparatus for the preparation of diorganozinc compounds
A 2009 review noted that diethyzinc is the cheapest available organozinc compound and that according to SciFinder it accounts for 60% of all references to diorganozinc reagents. The remainder coming from dimethyl (21%), dibutyl (4%), diisopropyl (4%), diphenyl (5%) and others (6%).
The different methods for preparing diorganozinc compounds can be grouped into four general approaches:
"I cut off the upper part of the tube in order to try the action of water upon the solid residue. On pouring a few drops of water upon the residue, a green-blue flame, several feet long, shot out of the tube, causing great excitement among those present. Professor Bunsen, who had suffered from arsenical poisoning during his research on cacodyl, suggested that the spontaneously inflammable body, which diffused an abominable odour through the laboratory, was that terrible compound, which might have been formed by arsenic present as an impurity in the zinc used in the reaction, and that I might be already irrecoverably poisoned. These forebodings were, however, quelled in a few minutes by an examination of the black stain [which was zinc] left upon porcelain by the flame; nevertheless, I did afterward experience some symptoms of zinc poisoning."Dimethyl- and diethylzinc were the first available sources of nucleophilic alkyl groups, useful for the alkylation of inorganic and organometallic halides and of organic C=O and C=N-containing electrophiles. Early examples of the alkylation of metal-halogen functions were described by Frankland and others, and the dialkylzincs have been useful in this application ever since. Since they are less reactive than the comparable Grignard and organolithium reagents, they are particularly useful in the partial alkylation of element polyhalides, e.g., PCl3 → RPCl2.
In 1867 Butlerow, in Russia, prepared tertbutyl alcohol by reaction of 2 equiv of dimethylzinc with acetyl chloride on a large scale, using 250 g of dimethylzinc. In this paper, Butlerow disputes Frankland�s report of the toxic effects of dimethylzinc, saying that he and co-workers had worked with this compound for 5 years without taking any special precautions and never had a problem. He noted that smoking in the presence of dimethylzinc vapors (or, more correctly, vapors of the oxidation and hydrolysis products of dimethylzinc) changes the taste of the tobacco; it becomes unpleasantly sweetish!!
Following the discovery of the usefulness of Grignards in organic synthesis
there was a decrease in the studies on Zn compounds until about 1984 when
it was noted that enantioselective reactions could be performed using
diorganoZn compounds.
Addition reaction of organozinc compounds to carbonyl compounds.
The Barbier reaction (1899) is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol. The reaction is similar to the Grignard reaction but the crucial difference is that the Barbier reaction is a one-pot synthesis whereas a Grignard reagent is prepared separately before addition of the carbonyl compound. Barbier reactions are nucleophilic addition reactions that usually take place with relatively inexpensive and water insensitive metals or metal compounds in contrast to Grignard reagents or organolithium reagents. For this reason it is possible in many cases to run the reaction in water which makes the procedure part of green chemistry. On the downside organozincs are much less nucleophilic than Grignards. The Barbier reaction is named after Victor Grignard's teacher Philippe Barbier.
An example of the Barbier reaction is the reaction of allyl bromide with benzaldehyde and zinc powder in water: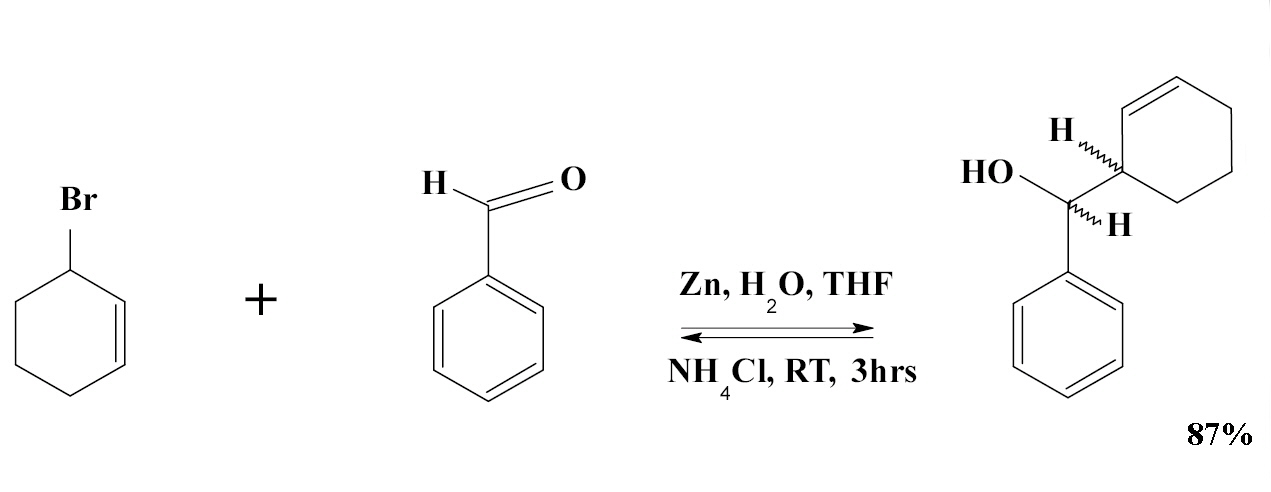
The catalytic enantioselective alkylation of aldehydes to afford enantioenriched secondary alcohols has been extensively studied since the 1980s. Diorganozinc reagents were the most widely used nucleophiles in this reaction, due to their low propensity to add to carbonyl derivatives without the presence of a suitable catalyst. A wide range of catalysts were investigated to optimise the conditions and conversion and to obtain the secondary alcohols in high enantiomeric excess.
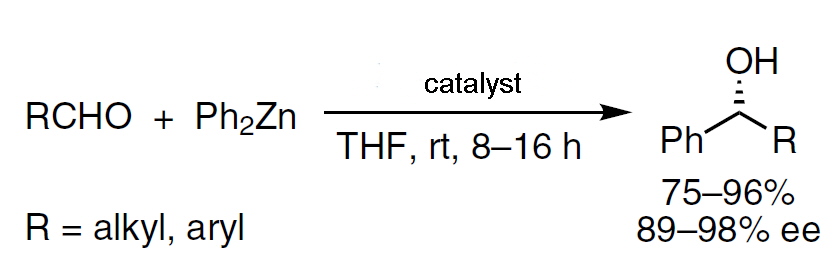
The carbon-tin bond is relatively stable and in the laboratory programme for this course the reaction of benzyl chloride with tin powder is investigated using either toluene or water as solvent. Both products ( R3SnCl and R2SnCl2) are found to be air stable. In 2009 a hydrate of the dibenzyl compound was reported. It was prepared using an unspecified mixture of water and toluene.
 Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,